Research
- Research Overview
- Organo Metallic Chemical Vapor Deposition
- Optical Response of Gold Nanoparticle Clusters
- Evanescent Wave Analytical Tools
- Surface Functionalisation
- Detection of Optically Active Molecules
- Optical Tweezers in Evanescent Fields
- Evanescent Microscopy and Spectroscopy
- Research Opportunities
- Research Group
- Former Coworkers and Students
- Collaborators
- Publications
Contact Information
Prof. Silvia Mittler
Physics & Astronomy 240
(519) 661-2111 x.88592
smittler@uwo.ca
FAX: (519) 661-2033
Surface Functionalisation
To functionalise a surfaces with specific physical, chemical or biological properties can have various reasons. One might think of engineered adhesion properties, designed hydrophilicity or hydrophobicity, reaction site creation on surfaces, biolocical recognition sites for sensors, photon bleaching stability, and lots and lots of highly innovative features.
There are various options to create a desired surface function, e.g.:
a) an existing surface is covered by an adlayer carrying the desired function
b) an existing surface is treated by a physical or chemical etching process to bare underlying desired functions
The group concentrates mainly on case a) were adlayers are fabricated by a variety of techniques. The adlayers may be spincoated out of a polymeric solution to gain film thicknesses in the order of 100 nm up to few micrometers, they may be produced by ion exchange reactions in an already excisting material, but they may also be fabricated in the Angstroem and nm-regime by Langmuir-Blodgett-Kuhn deposition techniques or by self-assembly routines. The last two techniques lead to monolayer formation and deposition.
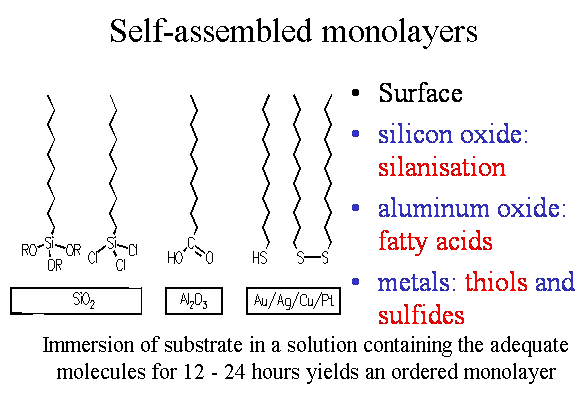
Self-assmbled monolayers are a unique technique to cover surfaces of various materials with exactely a monolayer. One has the choise of coating oxide surfaces with silanes, aluminium oxide surfaces with fatty acids and metal surfaces with sulfur based molecules, like thiols or sulfides. The self-assembly process sets in spontaneously by immersing e.g. a gold substrate overnight in a solution of thiols. Mixing of thiols with different functional end groups will lead to a mixed self-assembled film. The properties of this mixed film depends on various parameters of the two mixed species. So one can end up with a totally homogeneously mixed monolayer or a demixed system, where islands of one component are located in the matrix of the second component.
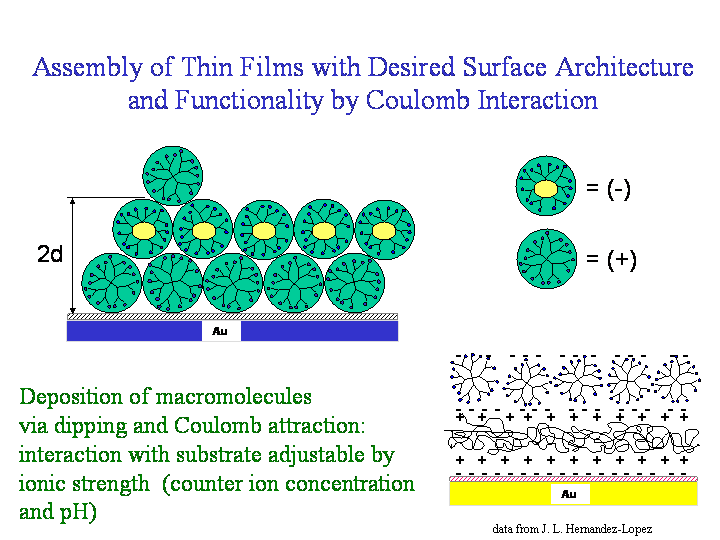
Another method to fabricate desired functionalities at surfaces is the assembly of charged species via Coulomb interaction. Here one has to use anions and cations in an alternative dipping procedure. The deposited amount of the charged material depends on the ion strength present. So the PH value, the concentration of the species itself as well as counter ion concentration play an important role here.
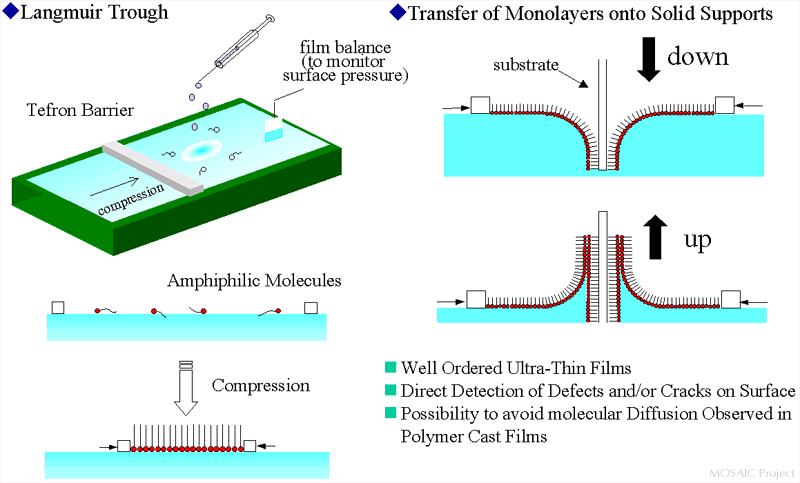
Amphiphile molecules - having a hydrophilic and a hydrophobic part - can be sprited on a water-air interface without being dissolved in the water. Due to the amphilicity these molecules locate themself right in the water-air interface. By reducing the available area successively the molecules will go through a gas analog phase, a liquid analog phase and a condensed phase. All of these phase transitions happen in two dimensions in a monolayered film on the water. This monolayer can be transfered into a solid substrate by dipping through it. Each dipping cycle transfers two additional monolayers to the substrate.
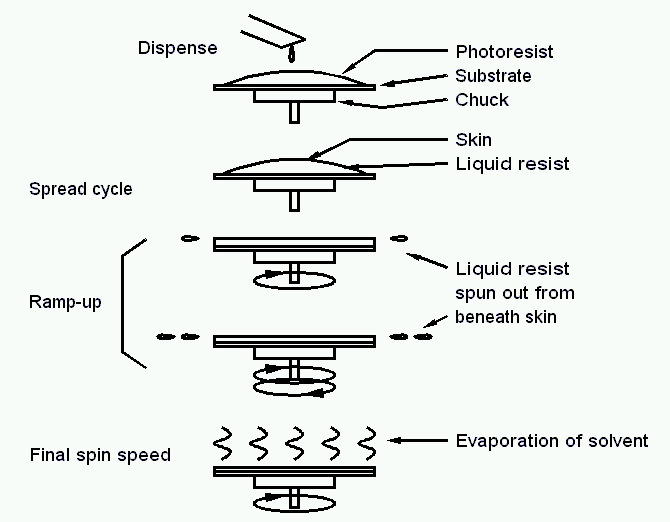
An easy way to form an adlayer on top of a flat surface is spin coating. Here the material which should be deposited is present in form of a viscous solution, which is applied onto the surface. Slow spining distributes the viscous material over the entire surface, whereas the qick spinning defines the final thickness of the material on top of the substrate. During spinning and also afterwards the solvent is evaporating. The figure above shows this procedure done with a photo resist. The sample is typically fixed to the chuck by means of a small vacuum.