ASSIGNMENT 1_DS - Chapters 4-8................... Assigned 9 Nov 2002 ...................Due date: Mon. 2 Dec 2002.
This assignment is designed ONLY for DISTANCE STUDIES students.
** Please note that all references to the text book refer to the sixth edition. If you do not have that edition, you will need to obtain a copy, or access it in your library. Not having the text is not an excuse for not doing the question. If it is absolutely necessary (and this will only happen if you provide me with a really good excuse), you can ask me to fax you the relevant pages.
1. Match the descriptions below to the letters (a) to (g). Some answers may be used more than once. You may consult your text book.
(i) A low, lumpy cloud layer that appears in rows, patches or rounded masses..... answer = (d) Stratocumulus.2. Answer the following multiple-choice questions. You may consult your text book.
(i) On a foggy night it is usually difficult to see the road when the high beam lights are on because of the (a) scattering of light.
(ii) Polar air is considered "dry" because the dew point temperatures are often quite low. However, the relative humidity of this cold, polar air is usually high because:
(c) the air temperature and the dew point are fairly close together(iii) When the air temperature increases, the saturation vapor pressure
(a) increases(iv) Condensation nuclei are important in the atmosphere because
(e) without them, condensation would not occur naturally in the atmosphere(v) When the air temperature decreases very slowly with height, and the environmental lapse rate is less than the moist adiabatic rate, the atmosphere is:
(b) absolutely stable3. Suppose there are 3 cities, which we will denote as city A, city B, and city C. The air temperature in city A is 40 degrees centigrade, in city B is 25 degrees centigrade, and in city C is 30 degrees centigrade. The dewpoints in each city are 25 degrees Centigrade for city A, 15 degrees centigrade for city B, and 20 degrees centigrade for city C. Use the graph in fig.5.12 (page 112 of your text) to determine the relative humidity in each city. Describe the procedure that you used.
Description of Procedure
(i) Determine saturated vapour pressure from ACTUAL air temperature by locating the air temperature on the abcissa ("x-axis"), and following vertically upwards until you encounter the red curve in fig 5.12. Then read across horizontally to determine the saturated vapour pressure.
(ii) Now determine the actual vapour pressure from the dew point Td. To do this, locate the "Dew-point temperature" on the abscissa of fig 5.12, follow up to the red curve, then read horizontally across to get the actual vapour pressure. (If the air was at temperature Td, the air would be saturated with water vapour.)
(iii) Now find Relative Humidity = (actual vapour pressure)/(saturated vapour pressure) x 100%
ANSWERS:...
City......................................................A...........................B.................................C............
---------------------------------------------------------------------------------------------
(a) T ...................................................40.........................25................................30............
(b) Td .................................................25.........................15................................20............
(c) Saturated Vap. Press......................73.........................31................................43............
(d) Actual. Vap. Press..........................31.........................17...............................24............
(e) Relative. Humidity....[31/73 x 100] = 42.5......17/31 x 100 = 54.9.......24/43 x 100 =55.8
4. On the Physics 103 web-site, under the section "on-line lectures", you will find an "adiabatic chart". Print off a copy of this. (If you want to save the file, simply "right-click" on the diagram, and choose the "save" option, and save it as a .gif file.) Then use the chart to describe the history of a parcel of air which has an initial temperature of 10 degrees centrigrade, and a dew-point of -5 degrees, if it is forced over a mountain of height 3.5 km and then descends down the other side. Indicate when the air will form cloud, and what the final temperature and dew-point will be when it arrives at the base of the mountain on the other side. [Hint: see your text book on page 174-175, which discusses the special focus section on "adiabatic charts", for an example]. Be sure to give both a written description and also indicate the relevant movements on the graph.
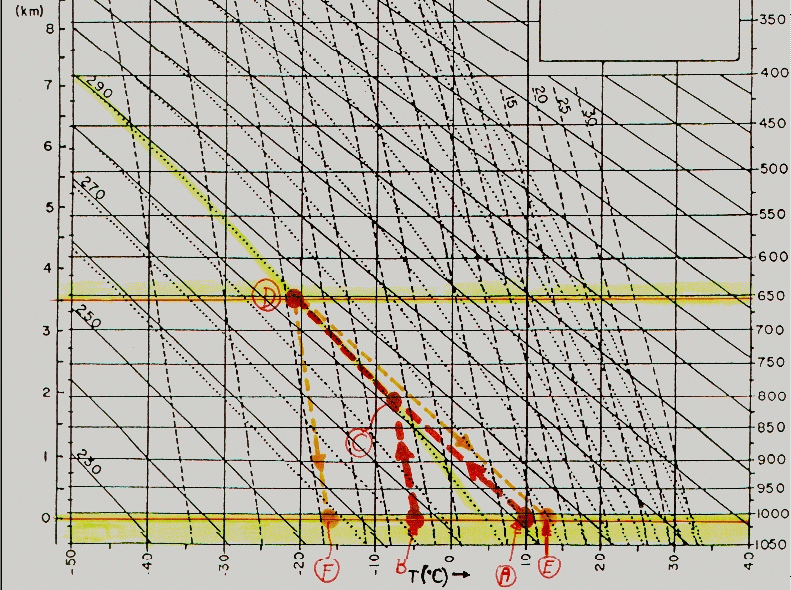
The attached graph (extracted from the "gif" file indicated in the question) shows the history of this air parcel. Note that 0km is at the TOP of the lower yellow band, NOT at the base of the graph. The parcel begins with a temperature of 10 degrees centrigrade, as indicated by the point A. It ascends up the mountain, decreasing in temperature at the dry adiabatic lapse rate, as indicated by the line from A to C (which is parallel to the "dry adiabatic" lines). At the same time, the parcel's dew-point is -5 degrees centigrade, as indicated by the point B.
As the parcel ascends, it's dew-point decreases, but less rapidly than its real temperature. The dew-point follows a slope parallel to the broken dashed lines, until it intersects the dry adiabatic line. This intersection occurs at C. At this point, the water in the parcel condenses out and it becomes a cloud. This point represents the base of the cloud, and is at an altitude of about 1.9km (also see the text book, 6th ed., page 172 - we expect the base of the cloud to be at a height of (10 - (-5))*125 = 1825 metres), so this is consistent).
The parcel then continues to rise but cools at the moist adiabatic lapse rate. This is indicated by the line CD (parallel to the yellow sloping dotted line which represents the moist adiabatic lapse rate).
Finally, the parcel reaches the top of the mountain at D, where-upon it descends down the other side. The parcel warms by a dry adiabatic process, thereby following the line DE. The dew-point decreases in a manner parallel to the broken lines, as indicated by DF.
Thus the final temperature of the parcel is at E, and equals approximately 13 degrees Centigrade. The final dew-point temperature is at F, and is approximately -16 degrees centigrade.
5. In the process of ice-crystal formation, ice crystals grow at the expense of surrounding water droplets (text book, 6 th ed., page 188). Explain how this occurs. Also discuss why water droplets exist at all in the cloud if the temperature is below 0 degrees centigrade. Keep your discussion to less than 350 words.
Water in a cloud is a mixture of vapour, water, and ice. It is especially important to note that even at temperatures below 0 degrees centigrade, not all water exists in the form of ice, and even at temperatures as low as -35 degrees centigrade there still exist water droplets and vapour. Spontaneous freezing occurs at around -40C. At higher temperatures, formation of ice particles requires special "ice nuclei", which are moderately rare - if these are too few in number, much of the available water remains in its liquid state. The water droplets are called "super-cooled" - they freeze if they encounter another solid object or an ice crystal, but stay liquid if they remain isolated. They do not spontaneously become ice crystals because small, spontaneously created clusters of ice crystal are destroyed by their own thermal motion. They need a certain "critical mass" before they can be large enough to overcome the effects of this thermal motion and actually begin to form ice-crystals, and this critical mass is greater at higher temperatures. Hence in all except the coldest temperatures, there are therefore generally far more supercooled water droplets than there are ice crystals.
Both the water droplets and the ice crystals are surrounded by vapour. The vapour pressure around each droplet and each crystal is determined by a balance between evaporation from the droplet or ice crystal, and re-condensation (in the case of droplets) or re-deposition (in the case of ice-crystals). Because molecules can escape from the droplet more easily than from the ice crystal, the vapour pressure around the droplets is higher than around the ice crystals. There is therefore a nett diffusion of vapour from the regions around the droplets to the regions around the ice crystals. This therefore increases the vapour pressure around the ice crystals and causes some of the vapour to deposit onto the crystals, causing them to grow. At the same time, the reduced vapour pressure around the water droplets causes evaporation from the water droplets. Thus there is a continual flow of water molecules from the droplets onto the ice crystals, so the ice crystals grow at the expense of the droplets.
Ice crystals may therefore grow in this way. As they get larger, they also collide with each other, which speeds up the process of growth. This collisional process is called aggregation. They may also collide with water droplets, causing freezing of the resulting water-ice collective, and this process is called accretion.