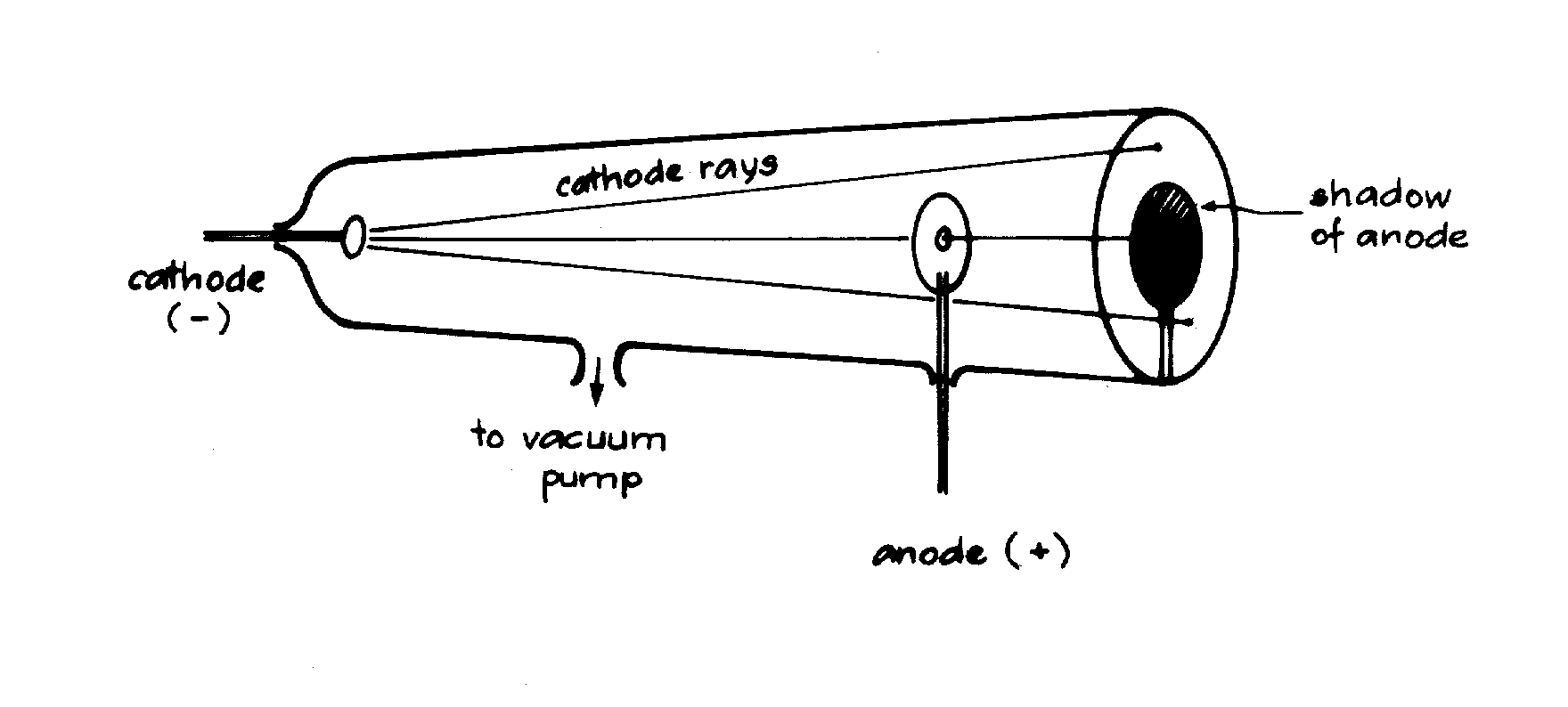
 slide1 ==> 2.1: A high voltage between cathode and anode causes "cathode rays" (electrons) to stike the glass
slide1 ==> 2.1: A high voltage between cathode and anode causes "cathode rays" (electrons) to stike the glass
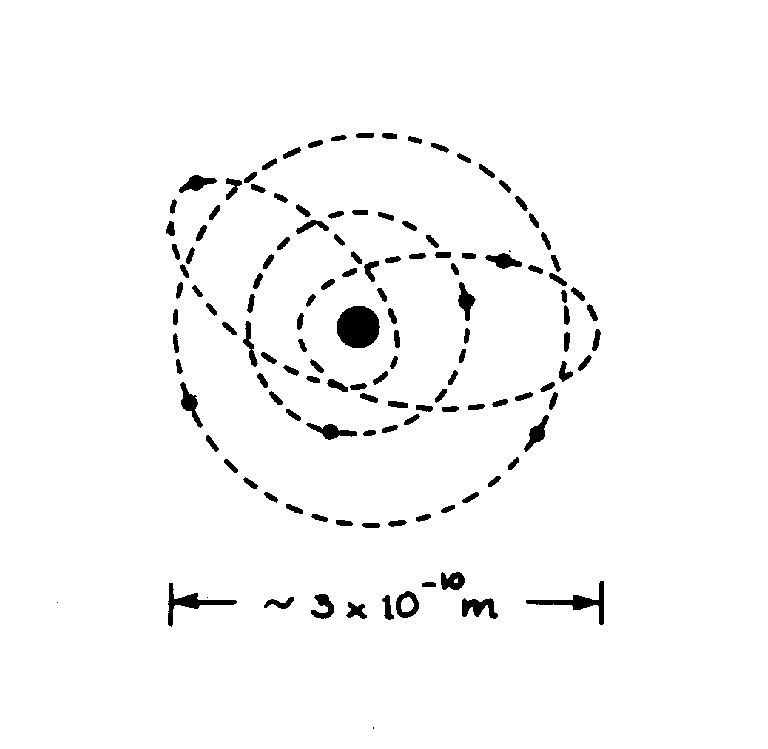 slide2 ==> 2.2: Sketch of a C atom. Electrons orbit the nucleus (heavy dot at centre) in shells of various sizes and shapes
slide2 ==> 2.2: Sketch of a C atom. Electrons orbit the nucleus (heavy dot at centre) in shells of various sizes and shapes
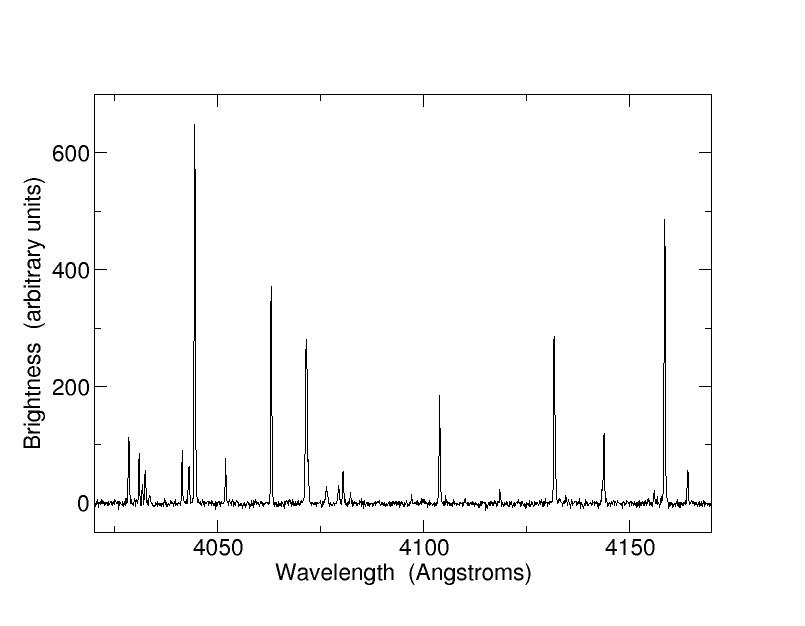 slide3 ==> 2.3: Light emitted from a heated gas of iron and argon. Only certain colours are produced
slide3 ==> 2.3: Light emitted from a heated gas of iron and argon. Only certain colours are produced
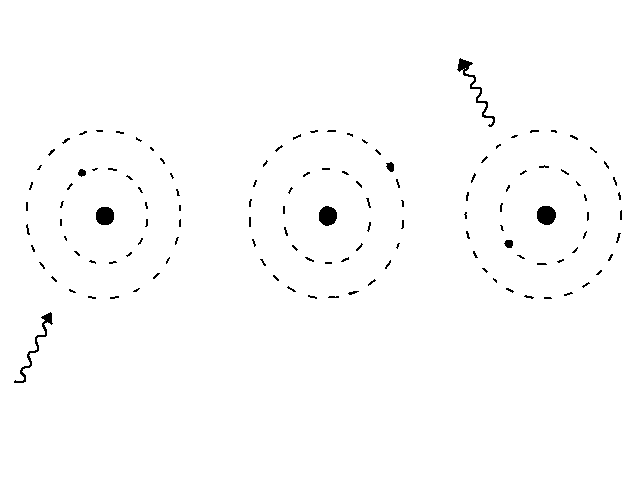 slide4 ==> 2.4: Absorbing a photon raises and electron to an orbit of higher energy; emitting one does the opposite
slide4 ==> 2.4: Absorbing a photon raises and electron to an orbit of higher energy; emitting one does the opposite
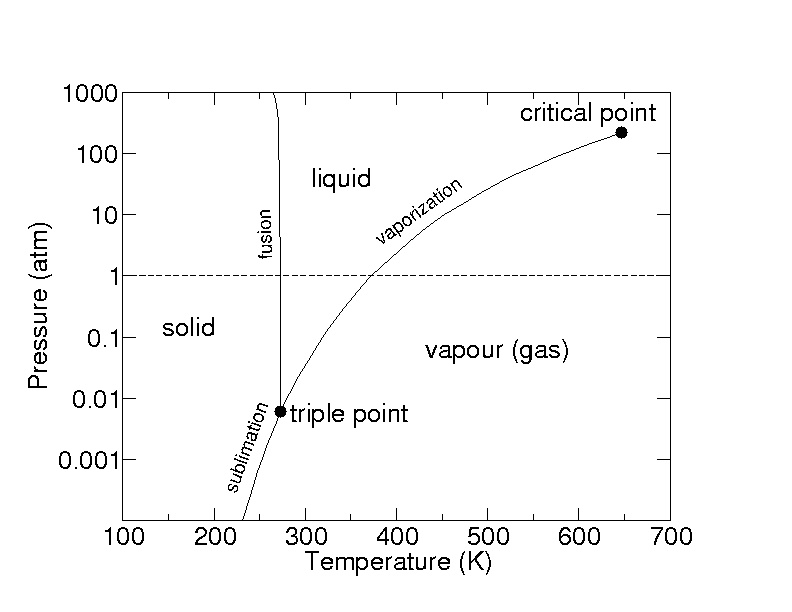 slide5 ==> 2.5: A pressure-temperature diagram (phase diagram) for water. Water at 1 atm pressure is on the dotted line
slide5 ==> 2.5: A pressure-temperature diagram (phase diagram) for water. Water at 1 atm pressure is on the dotted line
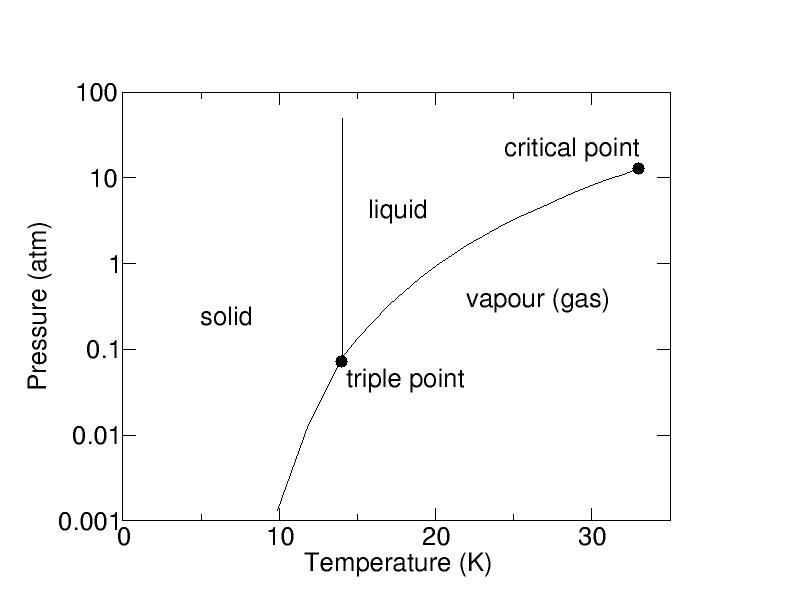 slide6 ==> 2.6: Pressure-temperature diagram for hydrogen molecules; all temperatures are far lower than for water
slide6 ==> 2.6: Pressure-temperature diagram for hydrogen molecules; all temperatures are far lower than for water
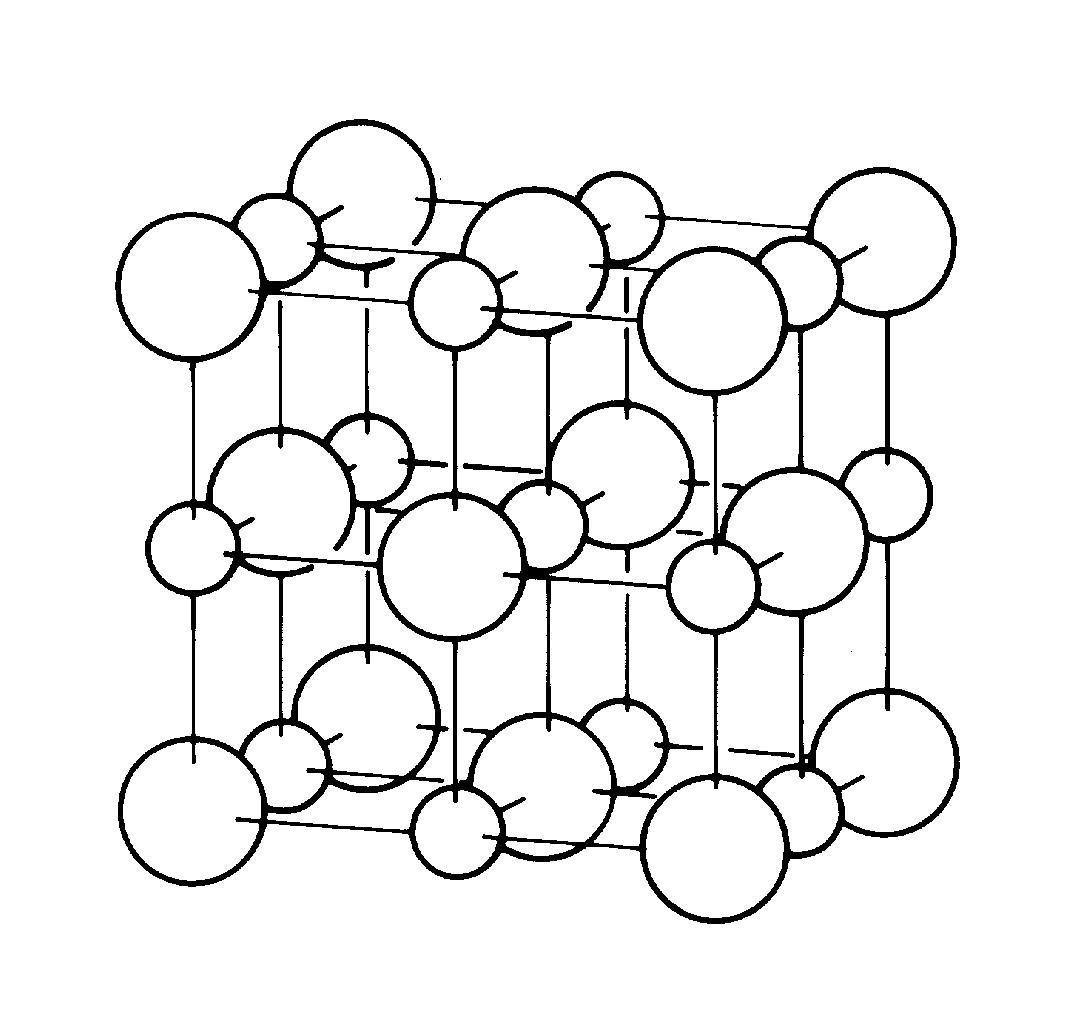 slide7 ==> 2.7: Cubic structure of salt (NaCl) crystals; Cl ions are larger than Na ions
slide7 ==> 2.7: Cubic structure of salt (NaCl) crystals; Cl ions are larger than Na ions
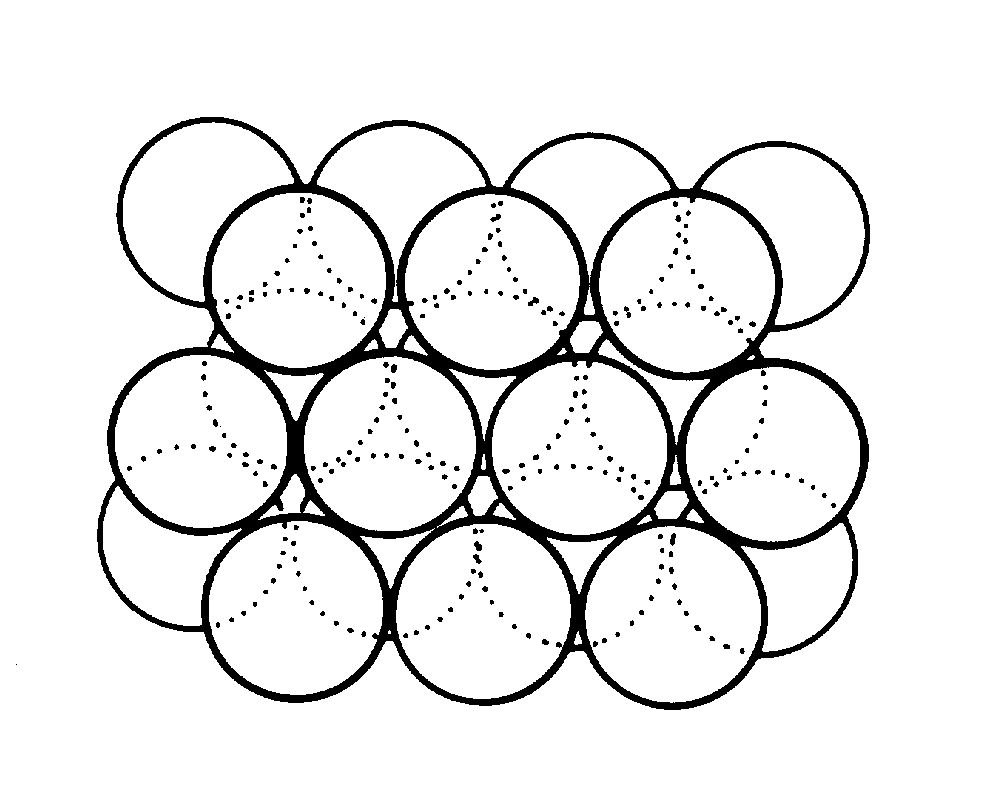 slide8 ==> 2.8: One of the arrangements for close packing of nearly identical ions or molecules
slide8 ==> 2.8: One of the arrangements for close packing of nearly identical ions or molecules
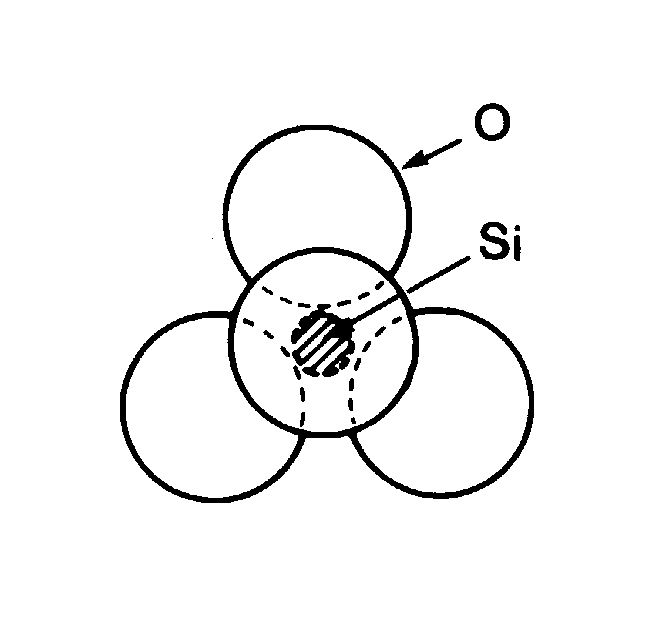 slide9 ==> 2.9: The tetrahedron of four O atoms and one Si atom, the basic structure of many silicates
slide9 ==> 2.9: The tetrahedron of four O atoms and one Si atom, the basic structure of many silicates
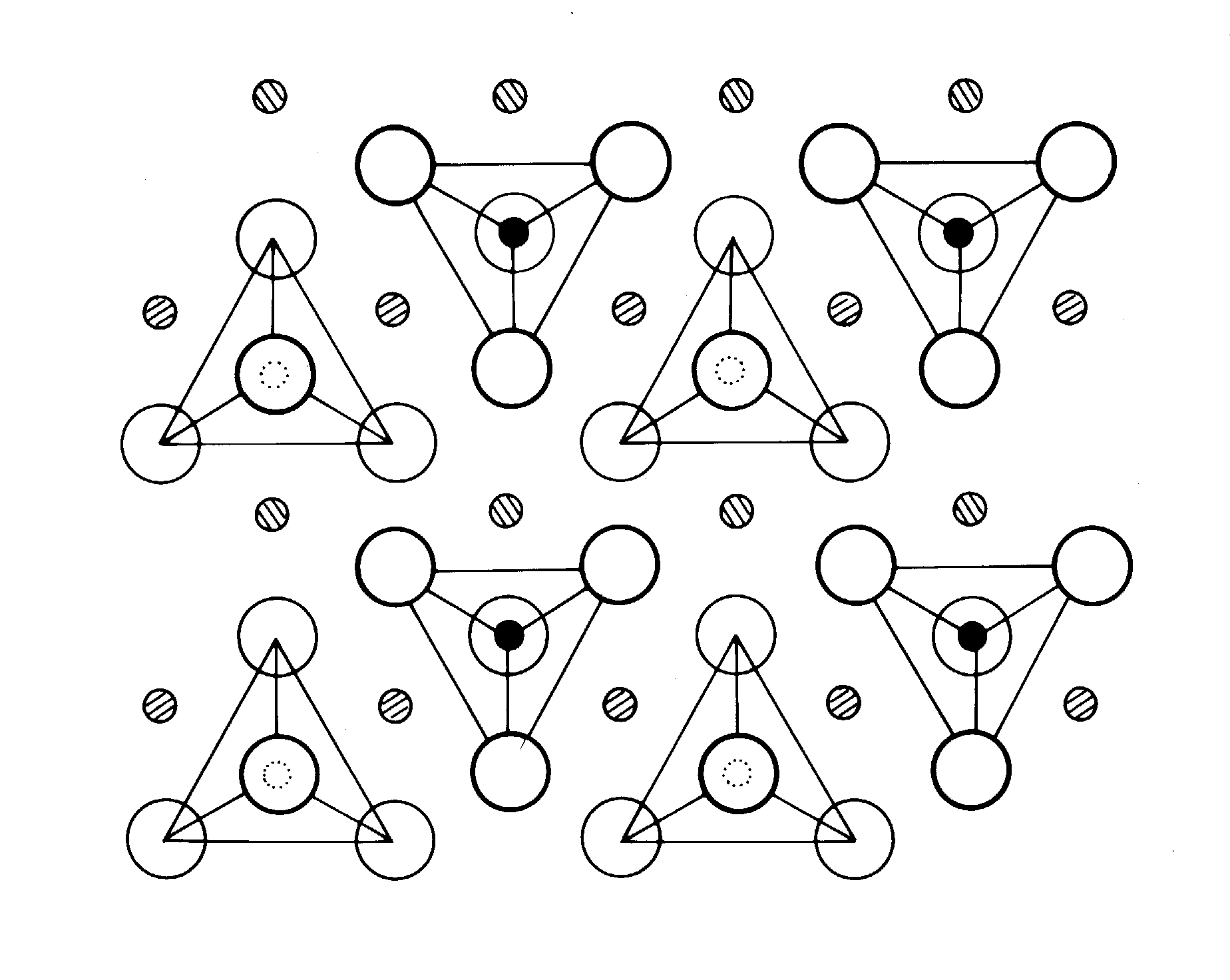 slide10 ==> 2.10: Crystal structure of olivine, with tetrahedra held together by Fe or Mg ions
slide10 ==> 2.10: Crystal structure of olivine, with tetrahedra held together by Fe or Mg ions
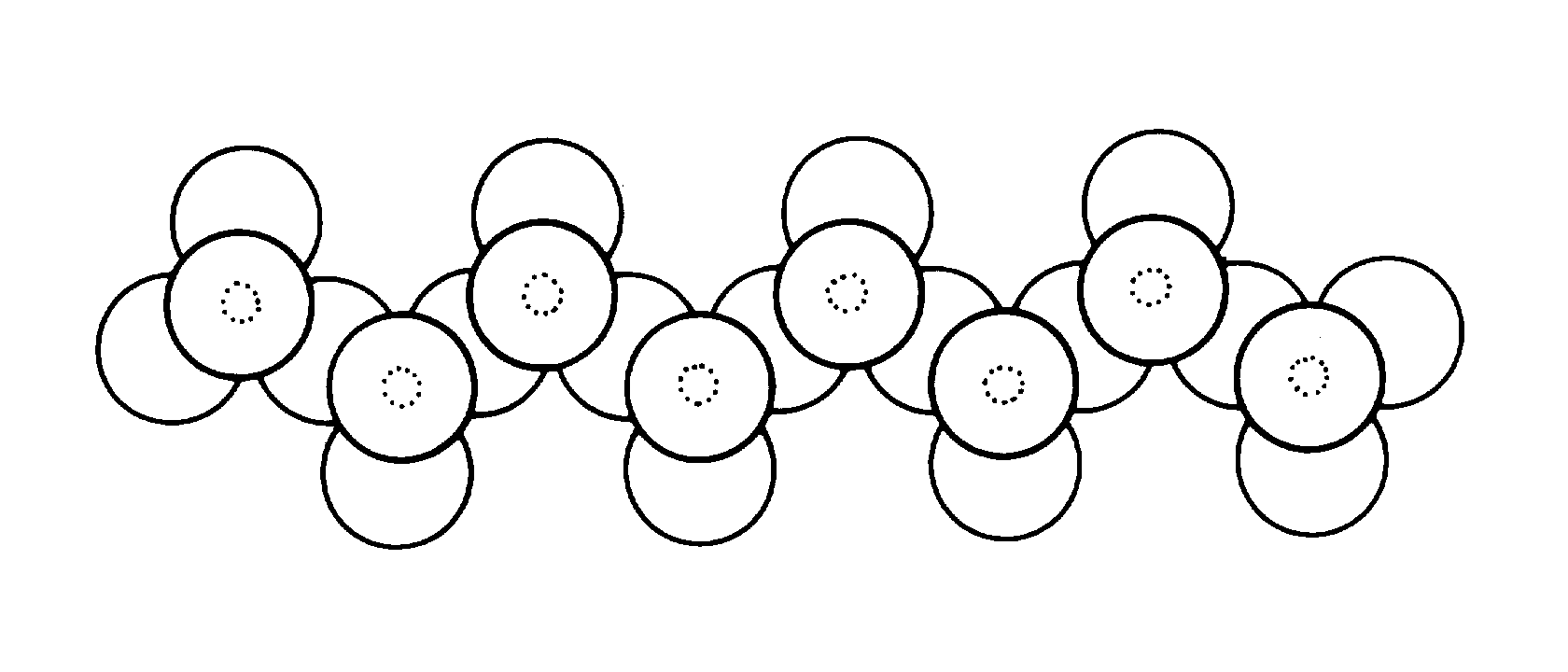 slide11 ==> 2.11: Crystal structure of pyroxenes is based on long chains of tetrahedra bound together by Fe or Mg
slide11 ==> 2.11: Crystal structure of pyroxenes is based on long chains of tetrahedra bound together by Fe or Mg
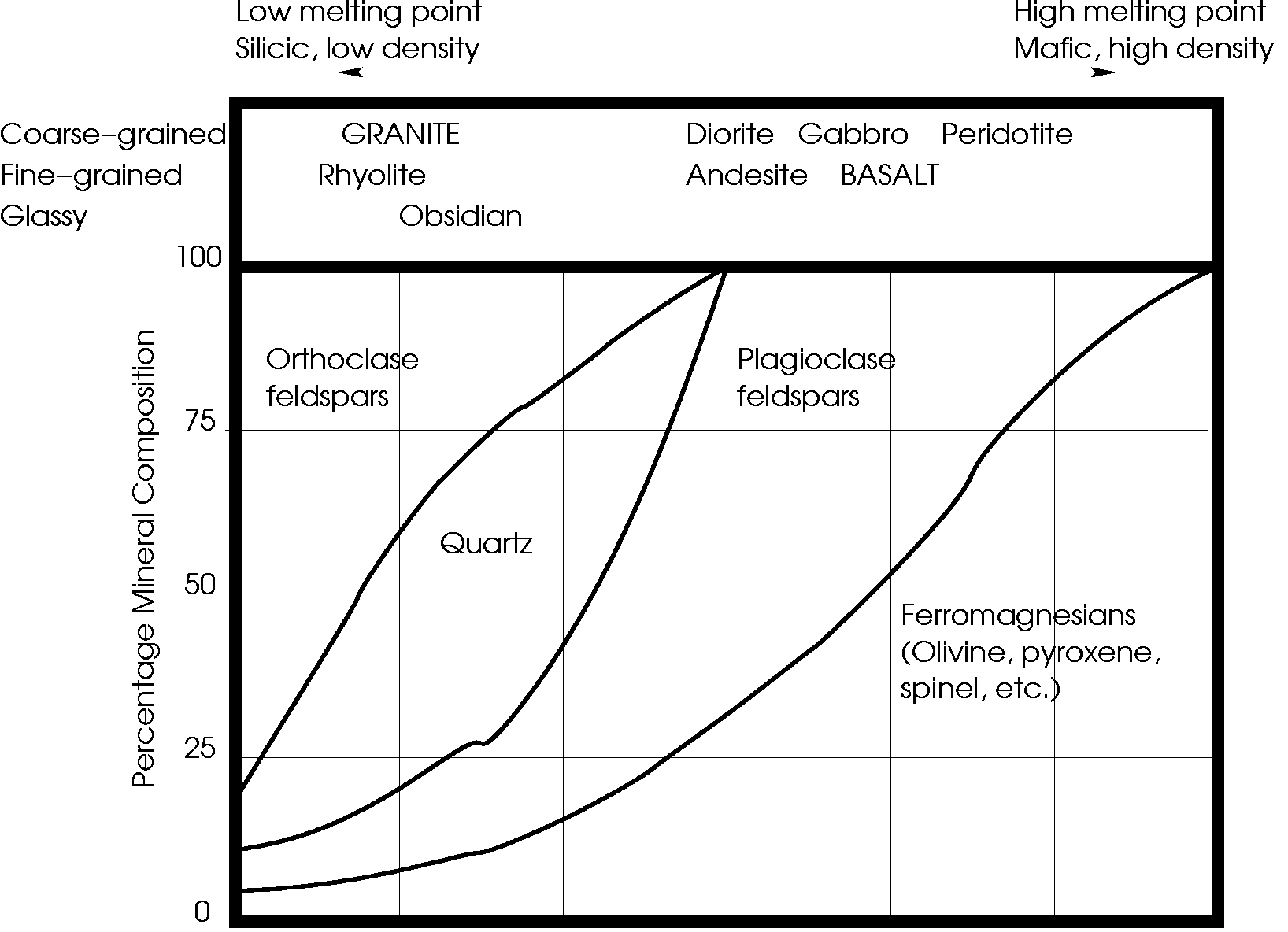 slide12 ==> 2.12: Mineral compositions and names of some common terrestrial rocks
slide12 ==> 2.12: Mineral compositions and names of some common terrestrial rocks